
This article was originally featured on Knowable Magazine.
Around the turn of the century, microbiologists at Danisco USA Inc. had a problem: The bacteria they used to make yogurt and cheese were getting infected with viruses. Investigating more deeply, the scientists found that some bacteria possessed a defense system to fight off such viral invaders.
These virus-resistant bacteria carried weird, repetitive collections of DNA letters in their chromosomes—bits of DNA from their encounters with past viruses that the microbes had “saved” in their own genomes. It was a form of molecular memory akin to the way that our own immune system remembers invaders so it can make antibodies against a recurring infection.
In this case, the microbes’ immune system, dubbed CRISPR-Cas, or more casually, just CRISPR, shreds any viral genome that matches the sequences in their molecular memory banks.
The yogurt-makers weren’t looking for biotechnology’s Next Big Tool. They just wanted to preserve the products in their vats. But other scientists soon realized the potential value of CRISPR for their own designs: With some modifications, CRISPR allowed them to cut any genetic sequence they wanted to, greatly easing the challenge of genetic engineering.
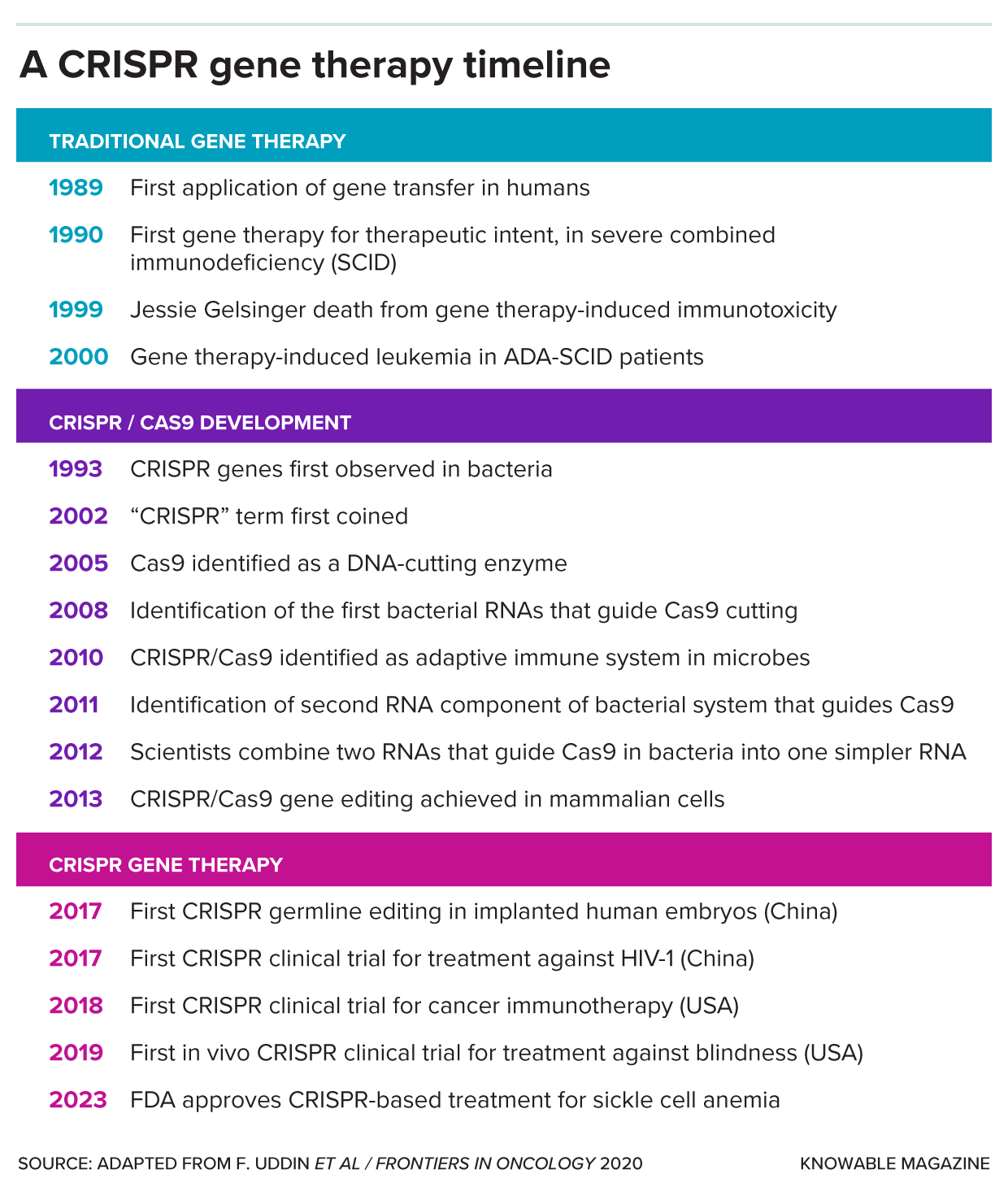
CRISPR systems have taken the biotechnology world by storm, nabbing a Nobel Prize and kicking off a new era of gene therapy. In December 2023, the US Food and Drug Administration approved the first CRISPR-based gene-changing treatment: a new gene therapy for the excruciatingly painful blood disorder sickle cell anemia.
“It’s been revolutionary for research,” says Kevin Esvelt, an evolutionary engineer at the MIT Media Lab in Cambridge, Massachusetts. “It’s accelerating all of biotech.”
Ever since scientists discovered CRISPR, there’s been a nagging question: Do similar gene-altering systems also occur in animals, plants and fungi—life forms known as eukaryotes, defined by the nucleus in which they store their genetic material?
The answer, now, is a definitive yes, according to a June 2023 paper in the journal Nature, authored by molecular biologist Feng Zhang and colleagues at the Broad Institute of MIT and Harvard in Cambridge, Massachusetts. The team found CRISPR-like DNA-snippers called Fanzors in an odd menagerie of eukaryotic critters, including fungi, algae, amoebae and a clam called the northern quahog (pronounced coe-hog).
Researchers hailed the finding as a fascinating addition to the CRISPR family tree. And the discovery raises more questions: What are Fanzors up to? Can they, too, make a splash in biotechnology? And might Fanzor and CRISPR be the tip of an iceberg’s worth of DNA-slicing systems awaiting discovery?
Here’s some of what we know about microbial CRISPR systems and the Fanzors-come-lately.
What is CRISPR?
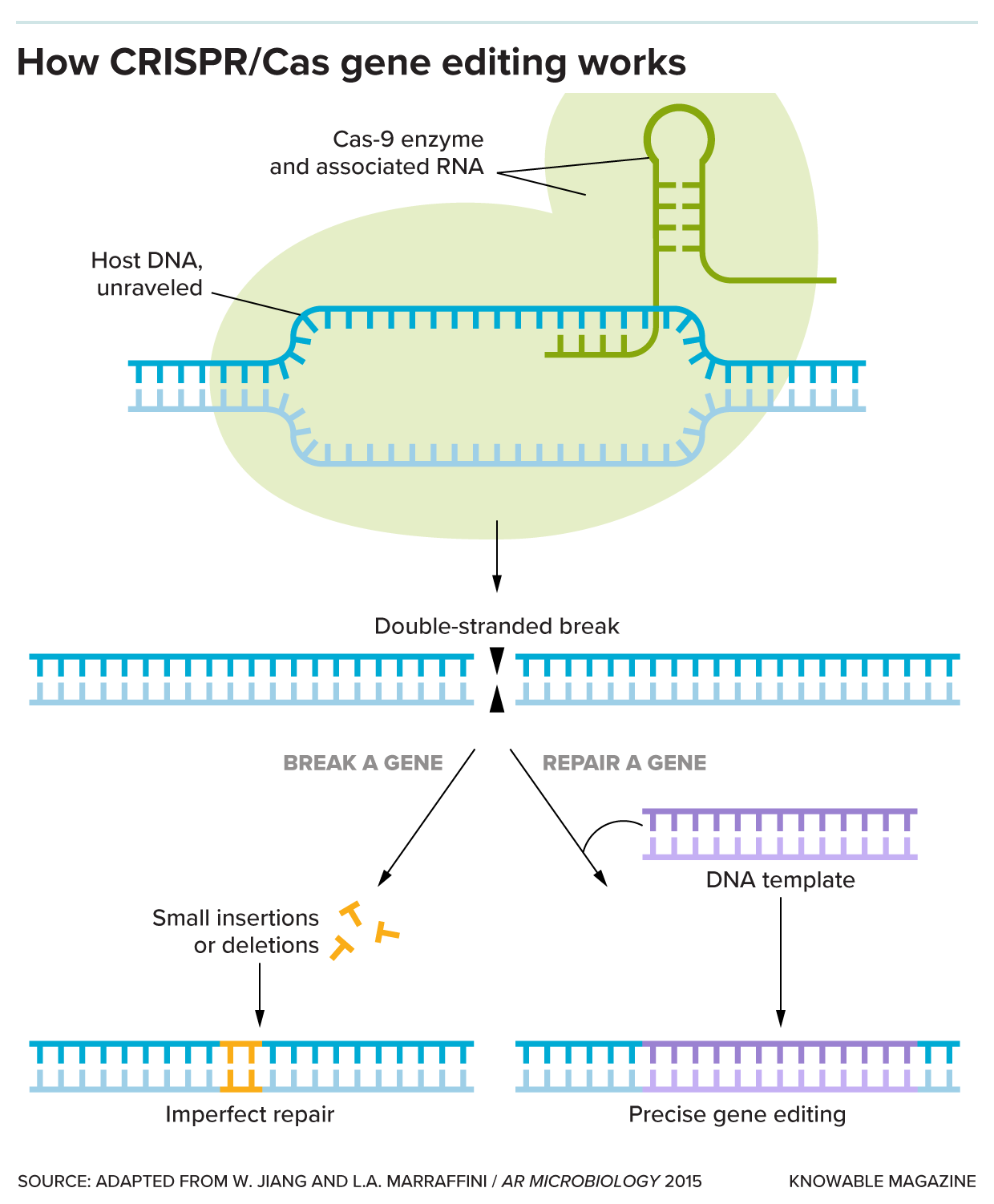
Just cutting DNA to bits isn’t a big deal. The special trick that CRISPR systems add is to make those cuts only in precise, targeted spots. This requires two elements: One is a guide to that location, a short piece of RNA that matches the target DNA sequence. The other is a protein, an enzyme to act as the “scissors.” Microbes have evolved a handful of scissor enzymes that make this cut, with names like Cas9 and Cas12.
When microbes are infected by viruses, the microbes collect small pieces of the viral genetic sequences and stow them together in a segment of their genome called CRISPR repeats. The next time that virus comes along, the microbe can use those sequences to create the guide RNAs. It can then use the enzyme scissors to snip up the genetic material of the virus and defend itself.
About half of known bacteria, as well as most of other microbes known as archaea, have CRISPR-Cas systems. Bizarrely, even some viruses have co-opted the CRISPR-Cas machinery, to fight back against microbes. So until the new study, eukaryotes were the only group totally left out of the fun.
What does CRISPR offer biotechnology?
Every living organism uses the same basic DNA code and proteins, so the CRISPR-Cas system can theoretically work in any organism scientists drop it into—though in reality, some tweaking is generally required.
The simplest application is to cut out undesirable DNA. Once the Cas enzyme cuts a target gene, the cell will glue the DNA back together, but imperfectly, creating a broken gene. For some applications, breaking a gene is all that’s needed, and it’s one possible approach to sickle cell gene therapy.
Sickle cell anemia occurs when the body has defective genes for the blood protein hemoglobin. But the body also has a separate hemoglobin gene used only by fetuses. Scientists want to reactivate the effective fetal hemoglobin in adult blood cells. There’s another gene, BCL11A, that keeps the fetal hemoglobin gene turned off. The new FDA-approved treatment uses CRISPR to break BCL11A, allowing the functioning fetal hemoglobin to spin back up in adult cells. The FDA also approved, in January 2024, this treatment for the blood disorder beta thalassemia.
Using a few extra molecular tricks, researchers can also use CRISPR to add a novel genetic sequence or to repair a broken gene.
Scientists are also trialing CRISPR-Cas-based therapies to treat diabetes; a form of amyloidosis; infections, including HIV; and a variety of cancers. And that’s not all. Researchers are applying CRISPR to turn pigs into possible organ donors for people, create better-quality, disease-resistant fruits, and eradicate mosquitoes that transmit malaria.
There are downsides. Breaking a strand of DNA in people is a risky business: If Cas cuts in the wrong place or the repair process goes wrong, the therapy could change the genome in a way that triggers cancer. Thus, many modern CRISPR-based approaches use modified versions of the Cas protein that don’t fully cut the DNA but edit it more subtly and safely.
How was Fanzor discovered?
Fanzor has been “hiding in plain sight,” says David J. Segal, a geneticist at UC Davis who wrote about the new age of genome engineering for the Annual Review of Genomics and Human Genetics in 2013. The Fanzor genes were first described that same year, but no one knew they encoded a Cas-like enzyme.
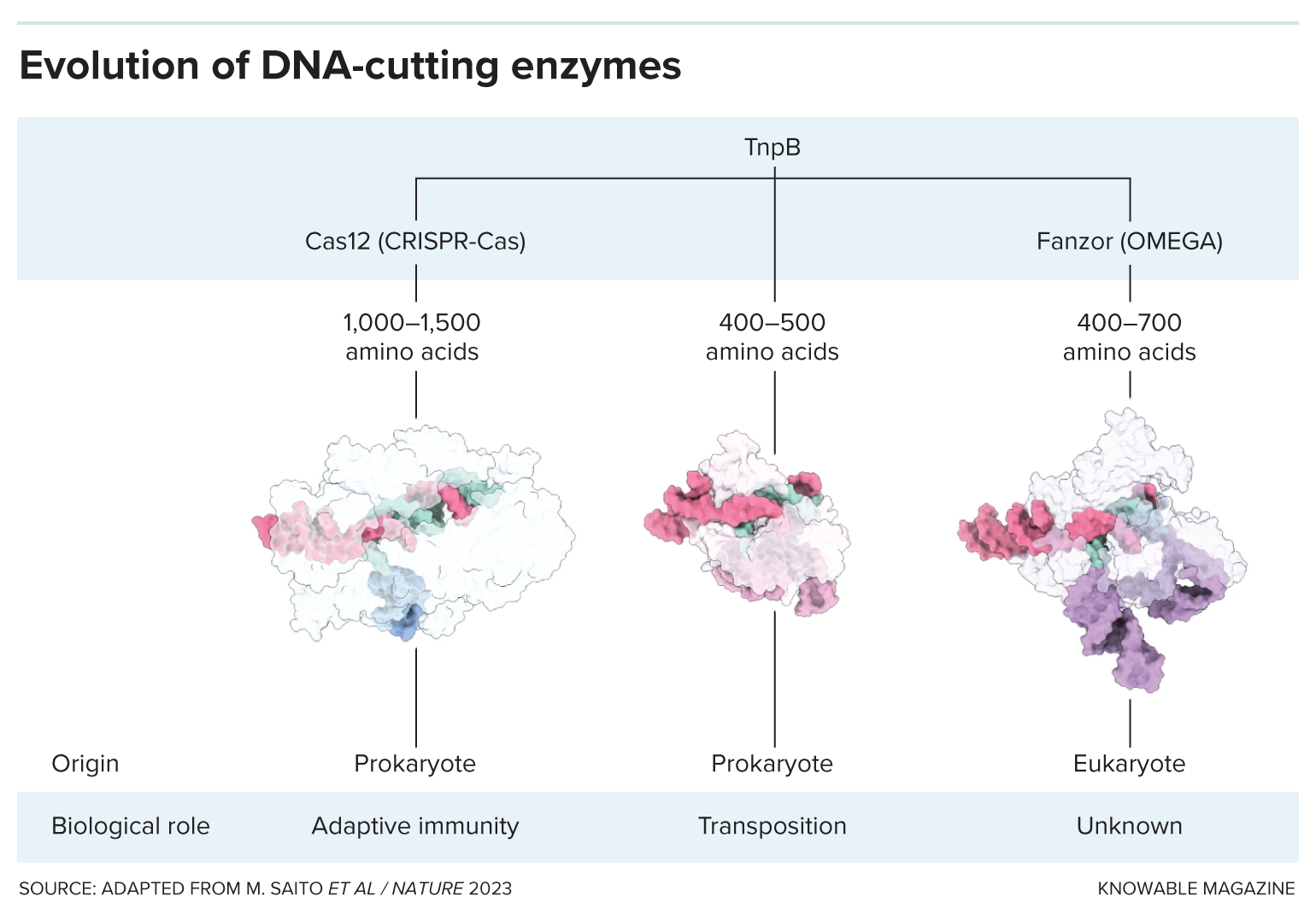
Weidong Bao, a researcher at the Genetic Information Research Institute in Cupertino, California, coauthored the 2013 Fanzor study. He and his colleague weren’t looking for DNA scissors; they were interested in what are commonly called “jumping genes.” These are DNA sequences that can hop out of one spot in the genome and reinsert themselves somewhere else. The most famous examples of these elements, known formally as transposons, are the jumping genes found to control kernel color in corn—work that led to a Nobel Prize for geneticist Barbara McClintock. In fact, about half of the human genome consists of transposons, but don’t worry: Most are no longer jumpers.
Bao knew that bacterial jumping genes often contain a gene of unknown function called TnpB. He went looking for similar genes and found them in the genomes of more than two dozen eukaryotes, including a fly, yeasts and molds, amoebas and several algae. The researchers named the eukaryotic version of this mysterious gene Fanzor instead of TnpB.
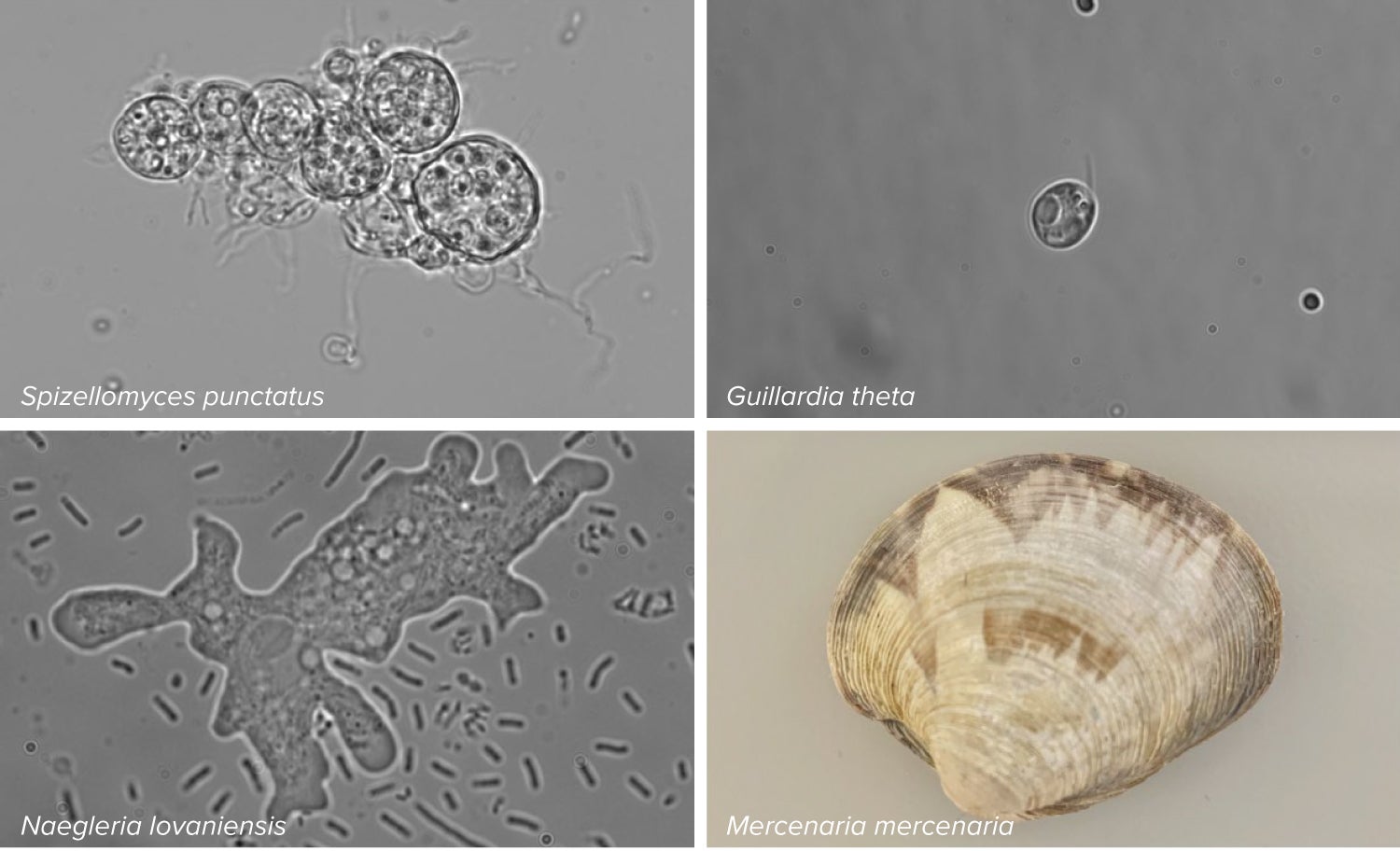
In a 2021 study, Zhang and his colleagues also stumbled onto Fanzor. They were building a CRISPR-Cas family tree, showing how these RNA-guided cutting systems had evolved, so they scanned genes and proteins from diverse organisms for similar components. They discovered that Fanzor is a cousin of the bacterial Cas genes, and that both gene families were descended from TnpB.
Are Fanzor genes involved in DNA-cutting like CRISPR-Cas systems are?
Zhang and colleagues reasoned that if the Fanzor genes and the Cas genes found in CRISPR systems are long-lost cousins, then Fanzor genes might also carry instructions for a protein that can cut DNA. Following that hypothesis further, the Fanzor protein might also use RNA guides to find and cut a specific DNA sequence.
So they tested it out by letting Fanzor genes loose in dishes of human kidney cells. The genes were translated to make Fanzor proteins. Then the scientists introduced guide RNAs to these Fanzor proteins and left them to stew for three days. If the Fanzor protein was a DNA-slicing enzyme, the cell would glue the DNA back together, but imperfectly, creating odd deletions or insertions at the sites matching the guide RNAs. That’s just what the team found.
Yep, Fanzor was a bona fide, RNA-guided, DNA-cutting enzyme.
Will Fanzor complement or supplant CRISPR-Cas in biotech?
Snipping a handful of genes in dishes is a long way from being biotechnology’s next big tool. So far, the best Zhang’s team has been able to do is to cut the target gene site 18.4 percent of the time, and they haven’t even tried to repair a faulty gene with it. That’s not nearly as good as CRISPR-Cas, which has the benefit of the years scientists have spent optimizing the system.
One potential advantage Fanzor has over CRISPR-Cas is its diminutive size. The Cas enzyme, plus a guide RNA, and potentially other needed elements, is a lot to deliver to a cell one wants to modify. Scientists often stuff those ingredients into a benign virus for delivery, but all the components won’t fit into the most commonly used viruses. The shrimpier Fanzor enzyme is a better fit. Still, scientists have already designed miniature versions of the Cas12 and Cas 13 enzymes that can fit into the viruses. So that’s a bit of a draw between the two.
And there’s a potential disadvantage to genetic engineering with Fanzor, says Esvelt. CRISPR-Cas systems usually require the guide RNA to match up with a DNA sequence that’s some 18 to 20 genetic letters, or bases, in length. Chances are, Esvelt calculates, there’s no more than one such perfect lineup in a genome. That means the Cas enzyme is unlikely to chop the genome anywhere except at the target site—good news for safe gene therapy. But Fanzor makes a match with just 15 DNA bases.
There’s a much higher chance, Esvelt says, that the smaller sequence might be repeated multiple times in a genome. Then Fanzor would cut the target site but also the other identical sites, which could spell disaster for gene therapy. Advantage: CRISPR-Cas.
The fact is, CRISPR works “just fine in most organisms,” says Sophien Kamoun, a plant pathologist at the Sainsbury Laboratory in Norwich in the United Kingdom who has reviewed the use of CRISPR to engineer crop plants. But Fanzor might still be useful for scientists working with certain bacteria where Cas enzymes are toxic, suggests Kamoun.
So overall, there’s no reason for most CRISPR-Cas labs to switch. “I’d rather use the thing that we know,” says Segal, who is working on treatments for neurodevelopmental disorders. “There’s enough mystery in what I do that I don’t need to worry about using a new enzyme.”
What do Fanzor systems do naturally?
Still, Fanzor is a very cool biological discovery. “It’s fascinating that there are CRISPR-like systems across so many kingdoms of life,” says Esvelt.
While we know what CRISPR-Cas systems do for microbes, it’s not clear exactly what Fanzor proteins do in nature. Researchers believe they’re somehow partnered with transposons—maybe helping them, or perhaps hitching a ride.
One possibility the Zhang group has considered is that Fanzor might create a defense system for the transposons. After all, a piece of DNA that can spring in and out of the genome, altering the DNA whenever it does so, is usually not good for a cell, so cells might fight back. Such a hypothetical Fanzor-based defense might work like this: A transposon would jump into spots in the genome that have the same genetic sequence that its associated Fanzor likes to cut. By jumping into that site, the transposon destroys the Fanzor target site, protecting the cell’s genome from being cut.
But if the cell were to remove the transposon, it would recreate the Fanzor target site, making its genome once again vulnerable to Fanzor’s snipping action.
In essence, Zhang speculates, the transposon would be telling the cell, “If you get rid of me, I’ll go somewhere else, I’ll make the Fanzor enzyme and it will come back and chop up the site you kicked me out of.” Better, then, to just leave the transposon where it is.
That’s just a possibility, though.
One organism that doesn’t naturally have Fanzors, Zhang says, is humans. But it remains possible that people possess some other, similar DNA-cutting system in the Fanzor-Cas family. Zhang’s group is actively hunting for new ones. “We’re trying to discover as many as possible,” he says.
This article originally appeared in Knowable Magazine, an independent journalistic endeavor from Annual Reviews. Sign up for the newsletter.
The post What’s going on with CRISPR gene editing? appeared first on Popular Science.
Articles may contain affiliate links which enable us to share in the revenue of any purchases made.
from Popular Science https://ift.tt/Gwknjt2




0 Comments