From cities in the sky to robot butlers, futuristic visions fill the history of PopSci. In the Are we there yet? column we check in on progress towards our most ambitious promises. Read more from the series here.
Decades before the first “test tube babies,” there were “biological cradles.” In fact, artificial wombs, which may soon be entering human trials for high-risk premature babies, got their start as far back as the early 1960s when researchers developed devices and methods to grow human embryos in a lab for as long as 50–60 days.
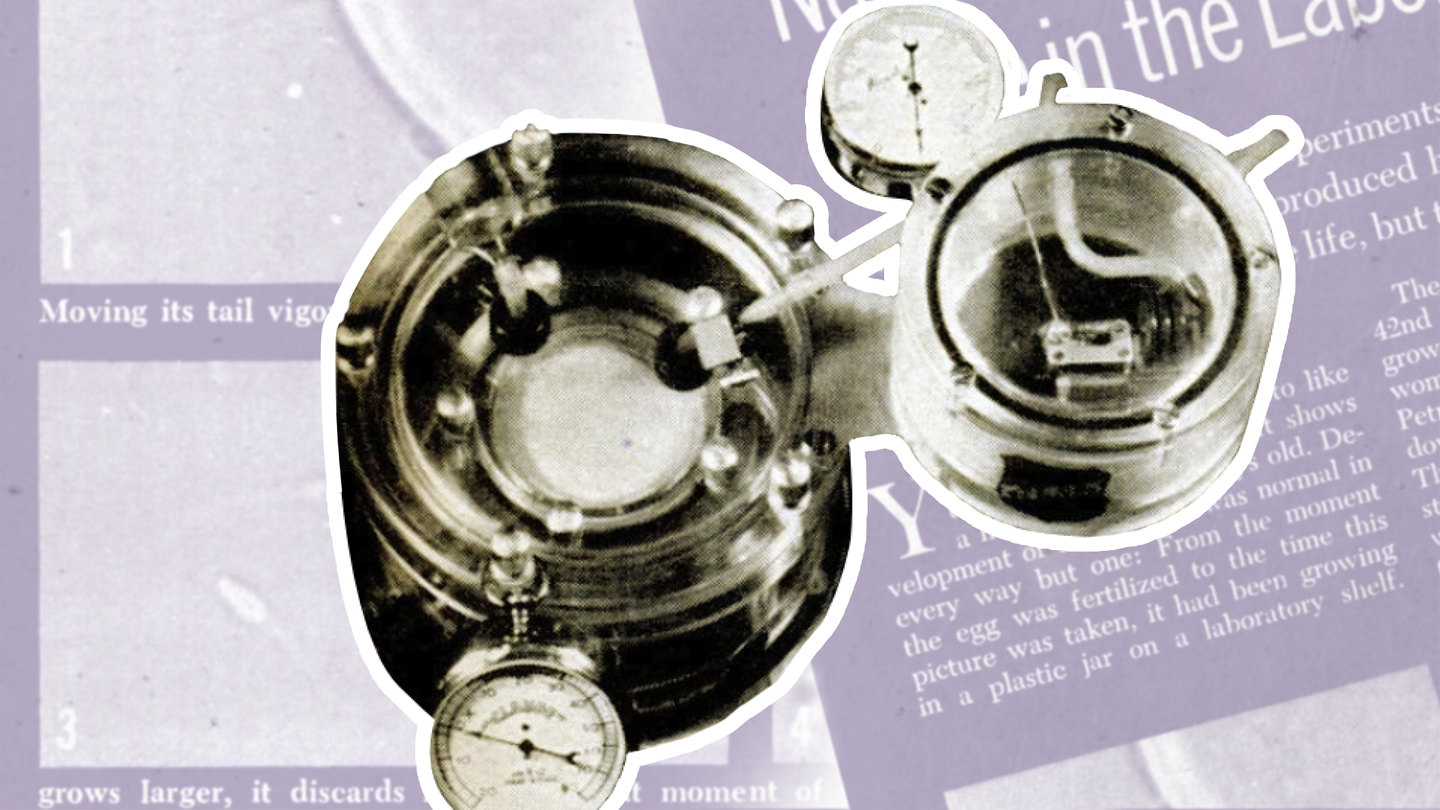
“This is not the relatively simple tissue culture,” Popular Science associate editor Joan Steen explained in June 1962, “where highly specialized cells like bone marrow or liver are kept growing in glass dishes fed by nutrient broths.”
Steen visited the lab of a surgeon in Italy, Daniele Petrucci, who had been developing embryos for their potential to support organ transplants. “This is growing the whole organism from scratch,” Steen wrote. “Taking the microscopic human egg cell and attempting, against all odds, to fertilize it and keep it alive for a long time.”
Much has changed in embryo research since 1962. From growing and printing synthetic organs to cellular reprogramming to artificial wombs, what scientists have learned from embryo studies has been breathtaking. But we still can’t sustain human embryos outside a womb for very long, and, more than a half century on, Petrucci’s vision of lab-grown organs remains mostly experimental.
Ectogenesis, the process of growing a human from conception to birth outside a body, is still the stuff of science fiction, ominously portrayed by Aldous Huxley in Brave New World, where lab-grown babies are engineered into social castes. In part, that’s because, by the 1970s, ethical concerns about embryo research led to funding restrictions, laws, and research regulations that have limited what scientists can explore. But it’s also because we have a long way to go in understanding the complex and nuanced placental interchange between the fetus and the pregnant person’s body.
“The challenge is the placenta does the work of a whole bunch of body systems during gestation,” says Michelle Oyen, Director of the Center for Women’s Health Engineering at Washington University in St. Louis. “You can’t just grow something independent of the vascular system, the lymph system, the nervous system—you need to have all of those connections, and that’s why it starts to become so complicated.”
The first successful “test tube baby,” Louise Brown, was born in 1978, paving the way for in vitro fertilization (IVF) as a solution for infertile couples. Since then, at least 12 million babies have been born using IVF, according to the International Committee for Monitoring Assisted Reproductive Technologies. But “test tube babies” are not actually grown in a lab for more than a handful of days before the fertilized egg is transferred to a uterus or frozen and stored for future transfer. (The procedure has recently become the focus of legal scrutiny after the US Supreme Court overturned Roe v. Wade in June 2022 and the Alabama Supreme Court ruled in February 2024 that frozen embryos are children.)
Brown’s birth signaled just how far scientists had come with embryo research, which set off alarms. A year later, in 1979, the US Department of Health Education and Welfare implemented the 14-day rule, prohibiting the growth of embryos in a lab beyond 14 days, citing ethical concerns. The restriction was widely adopted globally, limiting researchers’ ability to study later stages of embryonic development or to grow embryos for organ transplants.
As a result, human embryo and fetal development between 14 days and viability (roughly 22–24 weeks) is a kind of black box that scientists have been trying to explore in other ways. At the Center for Women’s Health Engineering, Oyen applies engineering tools and techniques to study that crucial developmental period about which so much remains unknown.
“I do a lot of computational modeling and image-based analysis,” Oyen explains. She also uses organ-on-a-chip models, which involves growing miniature tissues, or organoids—like placentas, lungs, hearts, even brains—on the type of chip that resembles a computer chip, carved with microchannels used to deliver fluids necessary to maintain the tissue while also providing direct means to monitor tissue growth. Organoids have been useful for drug testing and organ-specific research but have yet to advance to the point of providing regenerated organs for replacement therapy.
[ Related: A ‘brain organoid’ biochip displayed serious voice recognition and math skills ]
To grow organoids—and organs—requires some form of stem cell, generally an embryonic stem cell, an adult stem cell, or an adult stem cell that has been reprogrammed to an embryonic state, also known as an induced pluripotent stem cell (iPSC).
Embryonic stem cells are the most versatile; they start as blank slates, capable of developing into any type of cell, such as brain, skin, or liver. It’s their versatility that makes them so attractive to scientists seeking regenerative cures. The field of epigenetics, which can be traced back to the 1940s, explores the extrinsic factors that influence how an embryonic stem cell develops.
It wasn’t until 1998 that embryonic stem cells were successfully isolated by scientist James Thomson at the University of Wisconsin in Madison. Thomson’s discovery led to further restrictions on embryonic stem cell research, especially in the US.
By the early 2000s, the quest for human embryonic stem cell lines to fuel research led to therapeutic cloning, which enabled scientists to develop embryonic stem cell lines without relying on fertilization or surplus eggs from IVF clinics. Using such approved embryonic stem cell lines, researchers have been able to grow artificial embryos, or embryoids, that don’t require a separate sperm and egg. Of course, the 14-day rule still applies, which means the embryoid needs to be destroyed before it can develop further, although exceptions have been suggested since embryoids lack potential to develop into a functional fetus.
The restrictions imposed between the 1970s and early 2000s helped spur other approaches to stem cell research, not for the goal of growing humans artificially, rather with an eye toward understanding the very early stages of human development and toward regenerative medicine, the kind Petrucci envisioned—using stem cells to grow or regrow organs, cure or prevent diseases, and even reverse aging.
Adult stem cells, for instance, are not bound by the same research restrictions that govern embryonic stem cells. Adult stem cells, like the kind found in cord blood (yes, stem cells in infant cord blood are considered “adult”) and bone marrow, have regenerative properties, but their potential is limited by their advanced developmental stage. They cannot be induced to develop into any other cell unless they are “reprogrammed” first.
Cellular reprogramming, coaxing one cell type to convert to another by reprogramming it into an embryonic state, was first attempted in the 1980s and later advanced by Nobel Prize recipient Shinya Yamanaka. Today, scientists can revert mature cells, like skin cells, back to their embryonic, or pluripotent state (such cells are known as induced pluripotent stem cells, or iPSCs), enabling them to start fresh, regrowing into different tissue, like liver cells.
Beyond the obvious and significant ethical concerns, Petrucci’s model of growing organs via an embryo—a very small clone with perfectly matched tissue—would have been seriously inefficient, like building an entire house just to use one room.
3D bioprinting, however, using stem cell-based bioinks, shows promise. Researchers have successfully printed a variety of human organs such as kidneys, blood vessels, bones, muscle and skin tissue, and even a functioning heart.
[ Related: Scientists have 3D bioprinted functioning human brain tissue ]
But Oyen thinks it will be a while before growing or bioprinting organs will be reliable, pointing out that “you can’t engineer things without having the science. And that means this black box of early development is a real problem in trying to correctly and successfully recapitulate development of an organ.” She notes that there have been modest commercial successes like growing skin tissue for skin grafts, but “getting to that point where we’re using individualized stem cells from individual people to make a tissue-engineered graft is still in the future.”
It has been more than half a century since Petrucci shared with Popular Science his efforts to grow embryos in a lab for the purposes of human organ replacement. Since then, embryo research has pushed the boundaries of science, ethics, and our social conscience. While significant progress has been made in understanding the behavior and potential of embryonic stem cells (and other stem cells), we still can’t engineer organs reliably.
The post The decades-long quest for lab-grown organs appeared first on Popular Science.
Articles may contain affiliate links which enable us to share in the revenue of any purchases made.
from Popular Science https://ift.tt/FxLpERs




0 Comments